Notes: A Brief History of Germ Theory
On why we should update our priors
In the 1840’s, the maternity clinic in Vienna General Hospital housed two wards. One of them had a monthly mortality rate that was regularly in the double-digits and could climb as high as 30%, while in the other ward, that number seldom exceeded 4%.1 The key difference between the two was germs and how they were transmitted, though this wasn’t understood at the time.
The high mortality ward was staffed by medical professionals, while the second division was run by midwives. This earned the doctors a rather nasty reputation:
“Many heart-rending scenes occurred when patients found out that they had entered the First Division by mistake. They knelt down, wrung their hands, and begged that they might be discharged… they would protest that they were really quite well, in order to avoid medical treatment, for they believed that the doctor’s interference was always the precursor of death.”2
To some extent this was true. The professionally trained medical staff of the first ward were a lot more likely to carry out examinations for laboring women – simply put, they touched the patient more. Now this would not have been that big a problem if the doctors and medical students refrained from performing autopsies before attending births. Hand-washing between procedures was not common practice, and so the staff became vectors of disease – spreading the deadly puerperal sepsis from cadavers to vulnerable mothers.
A doctor at the hospital, Ignaz Semmelweis, noticed this pattern3 and mandated hand-washing with a chlorinated lime solution. This dramatically reduced deaths in the high-mortality ward and validated his observational hypothesis that unseen “cadaver particles” were causing illness.
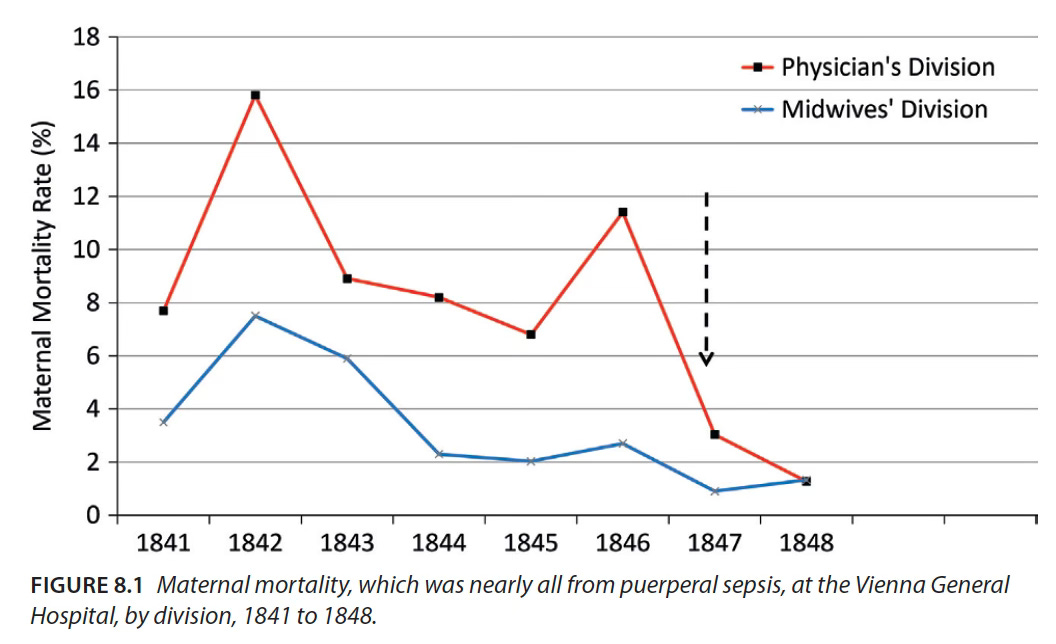
Shockingly, however, for his life-saving work, Semmelweis was removed from the hospital – he died in a mental asylum aged 47 while his superiors and colleagues remained convinced that the popular theory of miasma (“bad air” that supposedly effervesced from spoiled organic matter) was the true cause of maternal mortality. Moreover, Semmelweis was neither the first nor the last to face such rejection, and the medical community struggled with the particulate nature of infectious diseases for decades.
It was only by the end of the 19th century that there was some consensus. By then, a group of scientists that we are well familiar with (including Louis Pasteur, Robert Koch, and Joseph Lister) had collectively championed pathogenic microbes as the transmissible, causative agents of disease: germ theory. This late victory is despite the fact that by 1674, Anthony van Leeuwenhoek had already developed microscopes strong enough to see these tiny living organisms: “there were many small green globules. Among these there were, besides, very many little animalcules, some were roundish, while others, a bit bigger, consisted of an oval.”4
Looking at the broad sequence of events, it is truly astounding that about 200 years passed between the likes of Leeuwenhoek and Pasteur – footnoted by individuals such as Semmelweis who observed and worked against preventable loss of life. In this context, it is worth asking: Why did institutional medicine take so long to accept germ theory?
There doesn’t seem to be a singular factor that adequately explains this delay, and the scope here is quite large, both geographically and chronologically. However, there are a couple of threads that I found particularly compelling and interesting – perhaps examining specific historical moments can partly reveal how gradually, and at what human cost, our understanding of pathogens and disease prevention evolved.
Technological Hurdles:
One of the easiest explanations for a then-stagnating understanding of germs is practical: the microscopes just were not good enough. Early microscopes, such as those used by Robert Hooke, were essentially just very good magnifying glasses with limited power. They made objects about 20-50 times bigger which was far from sufficient to see what we consider “microscopic” today.5
Such was the state of viewing technology in Leeuwenhoek’s time. He was an interesting guy in that “he was not a doctor. He never published a scientific paper. He did not begin making contributions to science until he was 40. He did not invent the microscope.”6 Yet, today we associate his name with the invention because he was the first person to make advances that allowed us to view the truly minuscule – magnifying objects by 270 to 500 times their original size, giving us our first glance at “animalcules.” The key to this, according to some of his contemporaries, was the exceptional clarity of his glass lenses.
However, Leeuwenhoek jealously guarded this tacit knowledge: “my method for seeing the very smallest animalcules and minute eels, I do not impart to others... That I keep for myself alone.”7 His gate-keeping of methodology, combined with his limited scientific training, is one compelling explanation for why he failed to make the connection to early ideas of germ theory (such as Fracastoro’s theory of contagion)8 himself.
Elsewhere, progress on increasing magnification with a compound microscope was running into its own set of challenges. Here, Smrithi Sunil does an excellent job at explaining those issues: “van Leeuwenhoek’s single-lens microscopes had a problem: a tiny field of view. Early compound microscopes with two or more lenses promised to fix this, but the added optics created severe color aberration, which created a rainbow halo around every specimen because different wavelengths bent at different angles. In the mid-1700s, lens makers solved this by combining different glass types into the achromatic doublet, which canceled out the color fringing.”
All of this meant that the adoption of the microscope as part of the scientific toolkit was generally slow: “Some universities, including Leiden in Holland, had included microscopes among their teaching instruments, but medical instruction in microscopy appeared to actually decline during the 18th century.”9
This brings us back to Semmelweis, who never used a microscope to examine human tissue. His findings were mostly anecdotal. Similarly, a contemporary of his, John Snow, traced London's 1848 Cholera outbreak to a contaminated water pump (where a worried mother had washed her sick baby’s nappies); he, too, relied entirely on observing causal behavioral relationships rather than microscopic analysis. Here, again, while the authorities did take steps to mitigate the immediate disaster, popular belief reverted to the theory of miasmas.10 It would be another 35 years till Cholera would be considered a germ-based infectious disease following Robert Koch’s microscopic accounts from patients in Egypt and India in 1883: “It can now be taken as conclusive that the bacillus found in the intestine of cholera patients is indeed the cholera pathogen.”11
Institutional Hesitancy:
It makes sense that rigorous microscopic examinations helped move consensus. Koch was particularly good at this and had developed a methodology that helped him conclusively discover the causative agents of many infectious diseases including Anthrax and Tuberculosis. Today his process comprise the Koch Postulates: In essence, the hypothesized causative agent must be present in the tissue sample. Then it needs to be isolated in an uncontaminated culture, which can be injected into otherwise healthy animals. If these experimental models display signs of disease and if the same bacterium can be isolated from their bodies, then we can say that X germ is the cause of Y illness.
This methodology was crucial because simply observing microbes in infected tissue didn’t explain their origin:
“Were microorganisms to blame? If so, how did they get there? Many scientists held to the theory of spontaneous generation. If microorganisms could spontaneously appear, say, inside the human, how could a cogent theory of contagion even be considered? Dispelling the concept of spontaneous generation would be crucial for the development and acceptance of the germ theory of disease. Since spontaneous generation breached the church doctrine that God alone could create life, the debate even entered the metaphysical.”12
Koch’s postulates provided a solution to this dilemma. By demonstrating that microbes came from other microbes and not from thin air or non-living matter, his process made germ theory more palatable to both the scientific community and society at large. Moreover, it helped medical practitioners bypass uncomfortable theological questions about creation itself.
I bring up religion here because in some ways it might be fair to think of the rapidly professionalizing field of medicine as a sort of ‘secular ecclesiastical authority’ – one which worked to define itself against ‘irregular’ practitioners like midwives and folk/social healers. Like religious orthodoxy vs. heresy, ‘regular’ doctors (such as those ordained into professional societies and networks) claimed exclusive access to legitimate medical truth and worked to suppress competing traditions.
This is a far-reaching claim but there is documented evidence for this. Take, for example, the case of Martha Ballard, a midwife working in 18th-century America who kept detailed diaries of her practice. Her “ability to prescribe and dispense medicine made Martha a physician, while practical knowledge…as well as willingness to give extended care, defined her as a nurse. In her world such distinctions made little sense.”
Yet as medicine professionalized, these flexible, community-integrated practitioners faced systematic exclusion. As Laurel Thatcher Ulrich notes in A Midwives Tale, “professionals sought to be distinguished from the community they served (hence the title ‘Doctor’). Social healers, on the other hand, were so closely identified with their public that we [historians] can hardly find them.” This created not just professional rivalry but genuine resentment: “Eighteenth century physicians, like twentieth century historians, had difficulty distinguishing one social healer from another, yet they understood the power of their presence…female healers identified with the patients they served in ways male physicians could not. Little wonder that some physicians actively resented their presence.”13
This discord in, and need for, establishing the authority of professionalized medicine perhaps helps explain the continued resistance to germ theory. Going back to Semmelweis, we can see how a finding that suggested that doctors were directly responsible for their patients’ deaths would be both morally difficult to digest and serve as a devastating blow to the medical field. Many prominent practitioners at the time spoke out against Semmelweis, including the highly respectable Rudolf Virchow (i.e. the man who “essentially invented cellular pathology”). In-fact Virchow specifically “was thought to have said [on his death-bed] that, ‘If I could live my life over again, I would devote it to proving that germs seek their natural habitat: diseased tissue, rather than being the cause of diseased tissue.’”14 The fact that the dissenting faction included highly reputable practitioners who had contributed genuine scientific advances made it difficult to dismiss opposition to germs as mere ignorance.
Among men of science, it truly was the doctors who were most hesitant to accept microscopic pathogens as the cause of disease. We see evidence for this in John Drysdale’s 1878 book, The Germ Theories of Infectious Diseases. Here, alongside reconciling the many different theories on the cause of infections, he wrote that:
“in recent times the chief patrons of the [germ] theory, following Pasteur...have been natural historians and physicists, while in the medical profession, which is naturally more familiar with the clinical facts of disease, its adherents have hitherto been in the minority.”
Now maybe by this point the clinicians had just fallen prey to the sunk-cost fallacy, but it is clear that the formal medical community was more resistant to germ-theory than the scientists.
Practical Necessity:
Despite efforts to the contrary, we did reach consensus: slowly at first and then all at once. A telling example of this can be seen in how the events of the Franco-Prussian War (1870-71) played out.
During the course of the conflict the French military performed over thirteen thousand amputations for wounded soldiers with a success rate of just over 24 percent. About ten thousand amputees died because doctors used a dated technique by physician François Broussais, which included bandaging up the stumps with various salves – surgeons could just dip their hands into jars of ointment with their bare, unwashed hands as they moved from patient to patient. We can imagine how that would have been disastrous. In contrast, on the German side, they used the “antiseptic surgery” methods of Scottish surgeon Joseph Lister.15 By cleaning and wrapping the amputation wound in a mixture of carbolic acid (or phenol), linseed oil, and chalk “the Germans would be the first to lose more men directly to battle wounds than to subsequent infection.”16
They were not completely lucky however, and thousands of soldiers both on the German and French sides contracted infectious diseases in the clinics. These could result in sepsis, gangrene, or fever among others unpleasant symptoms with slim chances of recovery.17 Remarkably though, in field hospitals (such as those where a young Robert Koch was stationed) the sick patients were quarantined! There was a baseline understanding that the sick could infect the healthy.
This growing awareness was further supported by the work of Dr. Edwin Klebs, another frontline physician who conducted 115 autopsies during the conflict. His microscopic examination of tissue samples from individuals who died of infectious cases revealed that they “contained [in almost every case] the lower organisms that are referred to as bacteria, monads, etc.”18 Yet this evidence too, failed to settle the debate. Klebs’s bacteriological technique was allegedly lacking, leading even a sympathetic colleague to wonder: “Does disease follow bacteria, or do bacteria follow disease? We still don’t know the answer.”19
This is quite reminiscent of Virchow’s alleged statement on germs “seeking” out diseased tissue and representing the effects of an illness rather than its cause. Such push-back also makes sense because Virchow was indeed a firm opponent to Kleb’s findings, arguing against a pathogen first explanation. Christoph Gradmann explains the tension within the medical community particular well here: “Once Virchow had—to put it briefly— committed himself to the causation of inflammations by mechanical irritants and defined them as internal processes, any view that gave a biological explanation for the same phenomenon and at the same time assigned an important part to an exogenous pathogen was bound to represent a clear opposition to the dominant doctrine of the age.”20
Despite this ongoing scientific debate, practical necessity was clearly able to drive change on the battlefield. War made for rather clarifying venue and military leaders could not afford to be embroiled in academic debates:
“In the civilian world, the germ theory was a radical notion...But on the battlefield, such disputes were academic. Lister had demonstrated that his approach saved lives, so the Germans, keenly aware that disease had historically always been more deadly to military forces than actual battle, made Lister’s antiseptic procedure compulsory at all medical facilities.”21
This pragmatic acceptance of sanitation practices, even without full theoretical consensus, suggests that perhaps somewhere a silent majority understood the life-saving importance of sanitation (even if they struggled to explain why it worked and reconcile it with the dominant world-view).
Final Thoughts:
Today, it is odd to imagine a world that lacks the very mundane understanding that germs can cause disease. After all, we do practice basic infection control without really thinking about it: we wear masks when necessary, we wash and sanitize our hands, we filter our water and so on. This was not the case for most of human history where death from preventable diseases was part and parcel of life – as recently as 1900 “the three leading causes of death were pneumonia, tuberculosis (TB), and diarrhea and enteritis, which (together with diphtheria) caused one third of all deaths. Of these deaths, 40% were among children aged less than 5 years.”
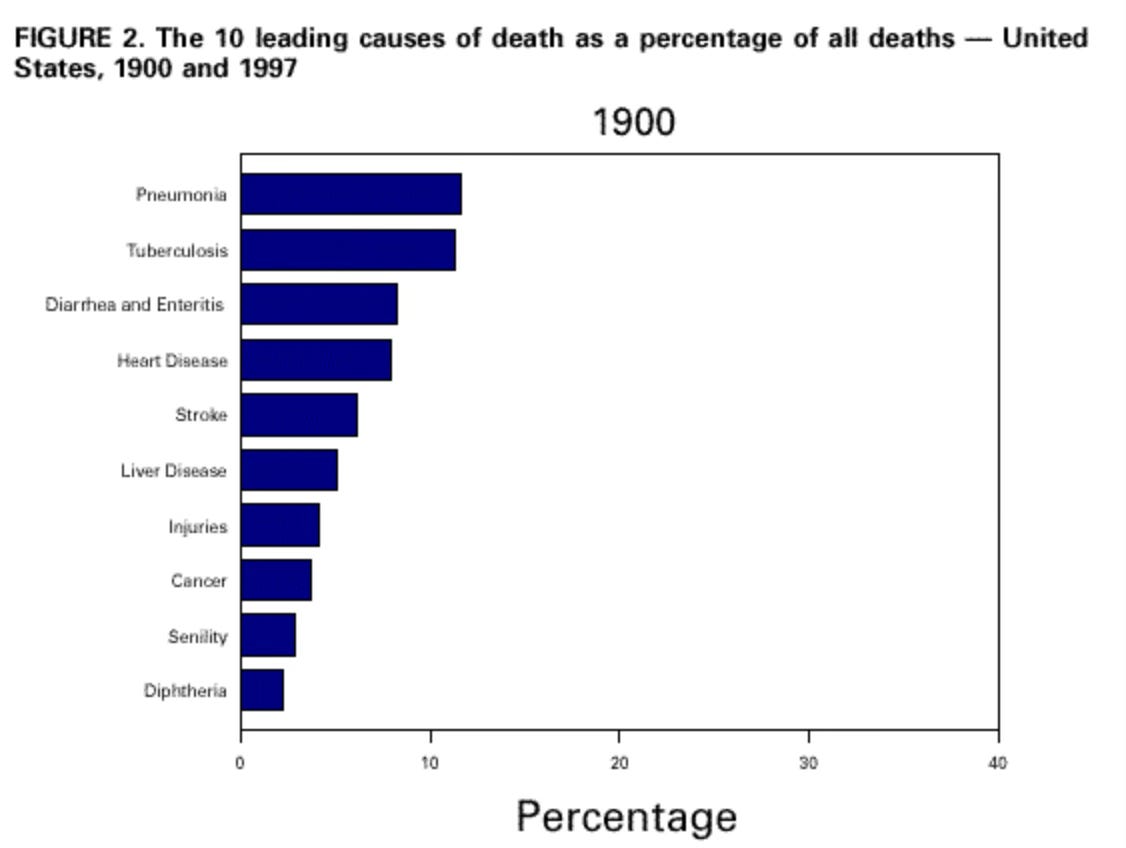
Just over a century later, this graph looks very different and germs are no longer the largest drivers of human mortality. Yet, as I have tried to demonstrate, we have travelled a long road to reach this point – circumventing technological barriers, moral discomfort, and institutional hesitancy. Taken together, these factors slowed down our collective scientific understanding and resulted in real, preventable loss of life.
Hindsight can be particularly illuminating and we can see that similar frictions exist in science today, that we still fail to update our priors as quickly as we should. However, crisis can force us to act: take for example the Covid-19 pandemic when emergency use authorizations fast-tracked mRNA vaccines that might otherwise have spent years in review. Sometimes we know things work but only feel comfortable adopting them as circumstances evolve.
In this light, the two-century journey towards modern germ theory feels like a necessary reminder of how our innate nature shapes scientific progress – and how that nature remains unchanged (unless rigorously challenged).
With thanks to Mike Riggs, Emma McAleavy, Lesley Gao, and Ariel Patton for their comments and feedback on drafts.
Cover Image: Antoine-Jean Gros, Bonaparte visitant les pestiférés de Jaffa [Bonaparte Visiting the Plague Victims of Jaffa] (1804), Musée du Louvre.
Suggestions for further reading:
Laurel Thatcher Ulrich, A Midwives Tale.
Thomas Goetz, The Remedy.
René Dubos, Louis Pasteur; Free Lance of Science.
Robert P. Gaynes, Germ Theory: Medical Pioneers in Infectious Diseases.
Semmelweis, The etiology, concept, and prophylaxis of childbed fever, tables 3 and 4 (pages 72 & 78)
Sherwin B. Nuland, The Doctors’ Plague: Germs, Childbed Fever, and the Strange Story of Ignác Semmelweis, page 85.
Semmelweis became even more certain when a close friend of his, Jakob Kolletschka, passed-away from an illness that was eerily similar to puerperal sepsis. Kolletschka had been nicked with a scalpel that was used for the autopsy of a woman who passed from “childbed fever,” and had thus contracted the infection himself. Semmelweis thus concluded that “cadaver particles” were being transmitted to the mothers on the unwashed hands of the physicians and students coming directly from the autopsy room.
Clifford Dobell, Antony van Leeuwenhoek and His Little Animals, page 110.
This number (of magnification of the first microscopes) depends on the source but the core point remains unchanged (in that it was insufficient).
Robert P. Gaynes, Germ Theory: Medical Pioneers in Infectious Diseases, page 57.
Dobell, page 144.
From Fracastoro’s De Contagione (as quoted in Gaynes op. cit): “The term contagion is more correctly used when infection originates in very small imperceptible particles. . . . There are, it seems three fundamentally different types of contagion: The first infects by direct contact only; the second does the same but, in addition, leaves fomes and this contagion may spread by means of the fomes. . .Thirdly, there is a kind of contagion which is transmitted not only by direct contact or fomes as intermediary, but also infects at a distance. ”
Gaynes, page 67.
Margaret Pelling, Cholera, Fever and English Medicine, 1825–1865, chapter 6. See also: “It [the Committee]. stated that it saw ‘no reason’ to adopt the scientific explanation of Snow.”
Thomas D. Brock, Robert Koch: A Life in Medicine and Bacteriology, page 160.
Gaynes, 81.
Laurel Thatcher Ulrich, A Midwives Tale, pages 58, 62, 65.
Susan E. Cayleff, A History of Naturopathic Healing in America, page 59.
Listerine, the mouthwash, was named after but not developed by, Joseph Lister.
Thomas Goetz, The Remedy, Chapter 1.
With some sources claiming that as little as one-in-fivet of these patients went on to recover: “The death rate in Koch’s day was about 20 percent.”
Christoph Gradmann, Robert Koch’s Medical Bacteriology, pages 42.
Brock, 30.
Gradmann, page 43.
Goetz, Chapter 1.



Great questions in here. A couple of thoughts.
One, I think that science is just really hard. When Leeuwenhoek saw microbes under his microscope, he had no idea that they caused disease. How could he have guessed? It's easy for us to see in hindsight that he had the first clue, but it was very nonobvious at the time.
And the truth can be weird. Imagine it's the 1850s and you're thinking about the germ theory. So… where do these germs come from? Are you telling me that microbes are everywhere, all the time? They're on our tables, in our food and our water? They're just floating around in the air, ready to land on us if we get an open wound? Are they in our bodies already? They're *everywhere*? That could be hard to believe. Indeed, Pasteur did a bunch of experiments to prove this.
Experiments and data can be hard to interpret. There are always confounding factors. Semmelweis had clear data to point to in the disease rates between the two wards, but as I recall one doctor pointed out that the ventilation systems were also different, so if you believed the miasma theory you could think that was the culprit. Florence Nightingale swore that she had seen one disease morph into another as it spread through a hospital ward—something the germ theory said couldn't happen.
Again, science is just really hard.
Another thing is that scientists have to not only discover knowledge but convince their peers of it. These are separate skills. Some people are good at both, like Pasteur. Some are not. I suspect Semmelweis was just bad at this. That doesn't excuse the doctors who refused to listen to him—and of course they were blinded by their own self-image and false pride—but it might help to explain what happened.
I think a real challenge here is that miasma theory (while completely false), like Ptolemaic theory, is actually fairly good at making practical and actionable predictions, especially pre-penicillin. If you take it seriously, you strive for good ventilation, you have open windows, doctors and nurses wear masks when treating infectious outbreaks, you prescribe exercise and good air for chronic conditions, people with allergic reactions should move around, etc.
Florence Nightingale's research demonstrated the importance of open windows and good sanitation, which is a result that is, of course, fully consistent with miasma theory.